Answer: The molarity of basic solution is 1M
Step-by-step explanation:
The chemical reaction for the reaction of KHP and NaOH follows
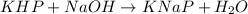
By Stoichiometry of the reaction:
1 mole of KHP reacts with 1 mole of NaOH.
Thus moles of acid = moles of base
Given :
moles of acid = [/tex]0.5\times \frac{100}{1000}[/tex]
moles of base =
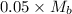
Equating the two :
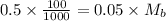

Thus molarity of basic solution is 1M