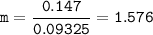The molality of solution = 1.576
Further explanation
Given
6.75% solution of ethanol in water
Required
The molality
Solution
Assume 100 g solution ,
Ethanol = 6.75 g⇒solute
Water = 93.25 g = 0.09325 kg⇒solvent
Molality = mol solute/kg solvent
mol Ethanol(MW=46 g/mol) :
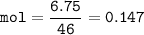
the molality :