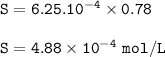The solubility of nitrogen in water at 25 °C= 4.88 x 10⁻⁴ mol/L
Further explanation
Given
78% Nitrogen by volume
Required
The solubility of nitrogen in water
Solution
Henry's Law states that the solubility of a gas is proportional to its partial pressure
Can be formulated
S = kH. P.
S = gas solubility, mol / L
kH = Henry constant, mol / L.atm
P = partial gas pressure
In the standard 25 C state, the air pressure is considered to be 1 atm, so the partial pressure of N₂ -nitrogen becomes:
Vn / Vtot = Pn / Ptot
78/100 = Pn / 1
Pn = 0.78 atm
Henry constant for N₂ at 25 °c = 1600 atm/mol.L=6.25.10⁻⁴ mol/L.atm
The solubility :