Step-by-step explanation:
In chemistry, a chemical formula is a scientific notation to show the chemical proportion of atoms that represents a particular compound or molecule. The empirical formula of a chemical compound illustrates the simplest whole number ratio between the elements contained in the compound whereas the molecular formula is the representation of the actual whole number ratio between each element of the compound.
Given that the empirical formula of carbamide is
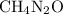 , then, the corresponding molecular weight (
, then, the corresponding molecular weight (
 ) is
) is
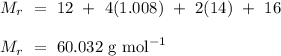 .
.
Subsequently, using the relation
![n \ * \[M}_(r)(\text{empirical formula})] \ = \ M_(r)(\text{molecular formula})](https://img.qammunity.org/2023/formulas/chemistry/college/zq6pa8pci80g7id09v0h918wp22tuzi0ui.png) ,
,
where
 is the multiple of empirical formula unit.
is the multiple of empirical formula unit.
Therefore,
