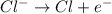Answer: The positively charged ions will be attracted to the negative electrode and gain electrons. Meanwhile, the negatively charged ions will be attracted to the positive electrode and release electrons.
Step-by-step explanation:
Electrolysis is breaking down of a substance into its constituents by the action of electrical current.
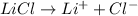
Cations wich are positively charged specie moves towards the negatively charged electrode that is towards the cathode. On reaching cathode, cations accepts electrons and get reduced through reduction reaction.
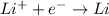
Anions wich are negatively charged specie moves towards the positively charged electrode that is towards the anode. On reaching anode, anions donate electrons and get oxidized through oxidation reaction.