The mass of 0.5 moles of the element : 60.241 g
Further explanation
Given
mass of 2 x 10²¹ number of atoms of an element is 0.4 g
Required
the mass of 0.5 mole
Solution
Find relative atomic mass of the element(Ar)
mol of 2 x 10²¹ atoms :
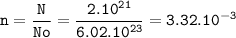
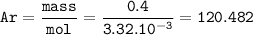
the mass of 0.5 moles :
