Answer:

Step-by-step explanation:
The formula for density is:
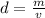
As mass and volume increase, so does the density.
In this problem, the density is the line. So, the steeper the slope of the line, the greater the density.
- Mercury (Hg) is the most dense because it has the steepest slope.
- Copper (Cu) is more dense than titanium but less dense than mercury.
- Titanium (Ti) is the least dense because it's slope is the least steep.
So, mercury is the most dense and titanium is the least dense.