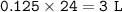a. 13.25 g
b. 3 L
Further explanation
Given
21 g of Sodium hydrogencarbonate
Reaction
2 NaHCO3 (s) → Na2CO3 (s) + H2O(l) + CO2(g)
Required
mass of residue
volume of CO2
Solution
mol NaHCO₃ (MW=84 g/mol) :
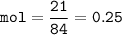
a. the residue = Na₂CO₃
From the equation, mol ratio NaHCO₃ : Na₂CO₃ = 2 : 1, so mol Na₂CO₃ :
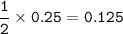
mass Na₂CO₃ (MW=106 g/mol) :
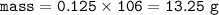
b. From equation, mol CO₂ = 0.5 x mol NaHCO₃ = 0.125
RTP= 25 C, 1 atm ⇒ 1 mol =24 L
So volume CO₂ :