The equilibrium constant, Kc=0.026
Further explanation
Given
1.72 moles of NOCI
1.16 moles of NOCI remained
2.50 L reaction chamber
Reaction
2NOCI(g) = 2NO(g) + Cl2(g).
Required
the equilibrium constant, Kc
Solution
ICE method
2NOCI(g) = 2NO(g) + Cl2(g).
I 1.72
C 0.56 0.56 0.28
E 1.16 0.56 0.28
Molarity at equilibrium :
NOCl :
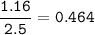
NO :
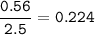
Cl2 :
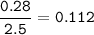
![\tt Kc=([NO]^2[Cl_2])/([NOCl]^2)\\\\Kc=(0.224^2* 0.112)/(0.464^2)=0.026](https://img.qammunity.org/2021/formulas/chemistry/high-school/e374tqfwu5vc012vc0a8i52y2ccec10hl8.png)