Answer:
=> 119.4597 g
Step-by-step explanation:
From our question, we have been provided with the molarity of the resultant solution.
We know that, molarity of a solution is contained in 1 L or 1000 cm³ of solution.
Hence we have 1.50 moles in 1000 cm³.
Also, we know that, 1 cm³ equivalent to 1 g for water.
Thus, from our question we have 995 cm³ of water.
Lets now solve our problem;
1.5 moles are contained in 1000 cm³
x moles are contained in 995 cm³
Cross multiply

1.4925 moles of NH4NO3 are required to be dissolved in water.
To find the mass of solute (NH4NO3) we use the formula;
Mass = RFM (Relative Formula Mass) x Number of moles
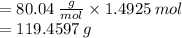
Therefore the mass of NH4NO3 needed to be dissolved in 995 g of water is 119.4597 g