Answer:
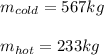
Step-by-step explanation:
Hello!
In this case, since equilibrium temperature problems involve the mass, specific heat and temperature change for the substances at different temperatures, we can write:
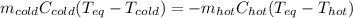
Thus, since we are talking about water and they both have the same specific heat, we can write:
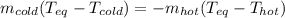
Now, we plug in the temperatures to obtain:
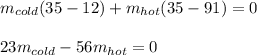
Next, since the total volume of water is 800 L, since it has a density of 1kg/L, we infer the total mass is 800 kg; that is why we can write a 2x2 system of simultaneous equations:
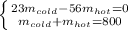
Thus, the masses of both cold and hot water turn out:
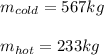
Best regards!