Answer:
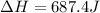
Step-by-step explanation:
Hello!
In this case, for this melting process, we can identify two sub-processes in order to take the stainless steel from solid to liquid:
1. Heat up from 298.15 K to 1673 K.
2. Undergo the phase transition.
Both process have an associated enthalpy as shown below:
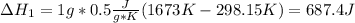
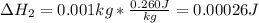
Therefore, the required heat is:
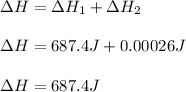
Notice the problem is not providing neither the mass or volume, that is why we assumed the mass is 1 g; however, it can be changed to the mass you are given.
Best regards!