Answer: The energy released when 4.81 mol of water freezes is 28.9kJ
Step-by-step explanation:
Latent heat of fusion is the amount of heat required to convert 1 mole of solid to liquid at atmospheric pressure or energy released when 1 mole of liquid freezes to solid at atmospheric pressure.
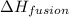 = enthalpy change for fusion = 6.008 KJ/mole
= enthalpy change for fusion = 6.008 KJ/mole
Energy released when 1 mole of water freezes = 6.008 kJ
Energy released when 4.81 moles of water freezes =
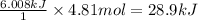
Therefore, the energy released when 4.81 mol of water freezes is 28.9kJ