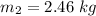Answer:
The value is
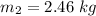
Step-by-step explanation:
From the question we are told that
The volume of the tank is
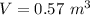
The mass of the Argon it contains is
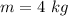
The initial pressure on of the gas is
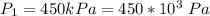
The initial temperature is
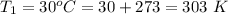
The new pressure inside the tank is
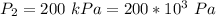
Gnerally given that the Argon remaining inside the tank has undergone a reversible, adiabatic process, then the final temperature of the Argon gas is mathematically represented as
![T_2 = T_1 * [(P_2)/(P_1) ]^{ ((k - 1 ))/(k) }](https://img.qammunity.org/2021/formulas/physics/high-school/u5yyushr6qlwkhbra3rypma0i97mwzvlhs.png)
Here k is the specific heat ratio of Argon with value
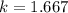
So
![T_2 = 303 * [(200)/(450) ]^{ ((1.667- 1 ))/(1.667) }](https://img.qammunity.org/2021/formulas/physics/high-school/ez3exoeikwxa9m7w2gyyhnpnr915dsmf05.png)
=>
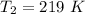
Generally from the ideal gas equation
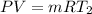
So
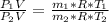
Here R is the gas constant with value
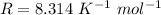
=>
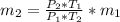
=>
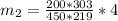
=>