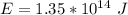Answer:
The value is
Step-by-step explanation:
From the question we are told that
The mass of matter converted to energy on first test is
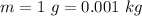
The mass of matter converted to energy on second test
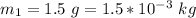
Generally the amount of energy that was released by the explosion is mathematically represented as
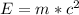
=>
![E = 1.5 *10^(-3) * [ 3.0 *10^(8)]^2](https://img.qammunity.org/2021/formulas/physics/college/u7cd3zh8qbwghx0pv7ckspipx52jvtevwn.png)
=>