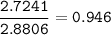mol fraction Codein : 0.054
mol fraction Ethanol : 0.946
Further explanation
Given
46.85 g of codeine
125.5 g of ethanol
Required
mol fraction
Solution
The mole fraction : the mole ratio of a substance to the mole of solution /mixture
mol Codeine, C₁₈H₂₁NO₃(MW=299.4 g/mol) :
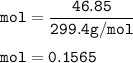
mol Ethanol, C₂H₅OH(MW=46.07 g/mol)
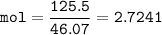
mol of solution :
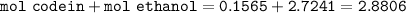
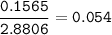
mol fraction Ethanol :