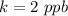Answer:
The value is
Step-by-step explanation:
From the question we are told that
The volume of the drinking water is
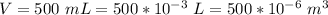
The mass of mercury it contains
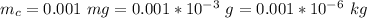
Generally the mass of water is mathematically represented as
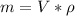
Here
 is the density of water with value
is the density of water with value
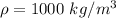
So
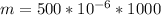
=>
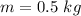
Generally ppb mean part per billon , and 1 billon is 100,000,000
So the part per billon which this represents is mathematically represented as
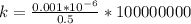
=>