Hey There!
_____________________________________
Answer:
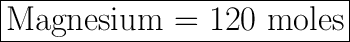
_____________________________________
Stoichiometry:
The study of relationship between the amounts of reactants and the amount of products in a chemical reaction as given by the balanced chemical equation of the reaction is called the Stoichiometry.
There are three types of stochiometric relationships,
- Mass-Mass Relationship
- Mass-Volume Relationship
- Volume-Volume Relationship
ASSUMPTIONS
To solve the stochiometric questions, we suppose,
- No side reactions takes place.
- Reactants are completely converted into products.
_____________________________________
There are two methods to solve the stoichiometric problems,
I prefer the moles method because it is easier to solve and the question is already given in the moles
_____________________________________

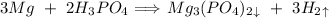
GIVEN DATA
Hydrogen gas = 120 moles
Moles of Magnesium = X = ?
SOLUTION
Since only the value of hydrogen is given and value of magnesium is asked so we will only focus on them only. Thus,
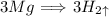
In the balanced reaction, there are 3 moles of magnesium used and 3 moles of Hydrogen gas are produced, thus The ratio of magnesium and hydrogen is 1 : 1. This means that Whatever amount of magnesium used, the same amount of hydrogen gas will be produced as the magnesium. This was the quick way to guess the answer but if you want to actually solve it here is how,
3 moles of magneisum produce 3 moles of Hydrogen gas
X moles of magnesium produce 120 moles of hydrogen gas
FOR X MOLES:
X =
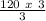
Simplify the equation,
X = 120 moles
_____________________________________
Best Regards,
'Borz'