Answer:
Dinitrogen trioxide.
Step-by-step explanation:
The correct name for the compound
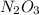 is dinitrogen trioxide. The compound
is dinitrogen trioxide. The compound
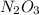 also happens to be a covalent compound, and the nomenclature of covalent compounds involves the usage of numerical prefixes such as "mono-" and "tri-".
also happens to be a covalent compound, and the nomenclature of covalent compounds involves the usage of numerical prefixes such as "mono-" and "tri-".
Since there are two nitrogen atoms in the compound
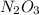 , we can use the numerical prefix "di-", meaning two, to signify that.
, we can use the numerical prefix "di-", meaning two, to signify that.
Since there are three oxygen atoms in the compound
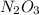 , we can use the numerical prefix "tri-", meaning three, to signify that.
, we can use the numerical prefix "tri-", meaning three, to signify that.