Answer:

Step-by-step explanation:
The formula we must use is given to us:
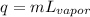
q is the energy, m is the mass, and L(vapor) is the latent heat of vaporization.
The energy is what we calculate and the mass is 2 kilograms. We need to find the latent heat of vaporization, which is on the table.
- We know the sample is copper.
- Find that element on the table, then the third box tells us it's latent heat of vaporization is 4730 kJ/kg
Now we know:
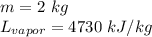
Substitute the values into the formula.
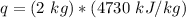
Multiply. Note that the kilograms (kg) will cancel each other out.
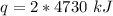
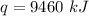
9460 kilojoules are required to vaporize 2 kilograms of copper.