Answer:
184.62 ml
Step-by-step explanation:
Let
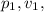 and
and
 be the initial and
be the initial and
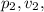 and
and
 be the final pressure, volume, and temperature of the gas respectively.
be the final pressure, volume, and temperature of the gas respectively.
Given that the pressure remains constant, so
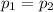 ...(i)
...(i)
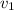 = 200 ml
= 200 ml
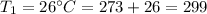 K
K
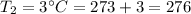 K
K
From the ideal gas equation, pv=mRT
Where p is the pressure, v is the volume, T is the temperature in Kelvin, m is the mass of air in kg, R is the specific gas constant.
For the initial condition,
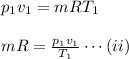
For the final condition,
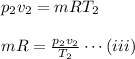
Equating equation (i), and (ii)
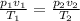
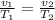 [from equation (i)]
[from equation (i)]
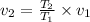
Putting all the given values, we have
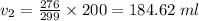
Hence, the volume of the gas at 3 degrees Celsius is 184.62 ml.