Answer: The initial volume of the gas is 7.72 L
Step-by-step explanation:
For an isothermal process the temperature is constant.

as P = pressure = 1 atm ,
V = Volume = 25 L
n = moles
R= gas constant
T = temperature
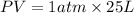
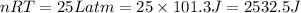 (1Latm=101.3 J)
(1Latm=101.3 J)
For isothermal reaction :
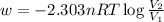
where , w = work done by system = -ve
n = moles = 1
 = final volume = 25 L
= final volume = 25 L
 = initial volume = ?
= initial volume = ?
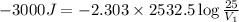
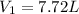
Thus initial volume of the gas is 7.72 L