Given :
The mass of the balloon was 1890 kg and had a volume of 11,430 m3 .
The balloon floats at a constant height of 6.25m above the ground.
To Find :
The density of the hot air in the balloon.
Solution :
We know,
Volume × ( Density of surrounding air - Density of hot air ) = mass
Putting given values in above equation, we get :
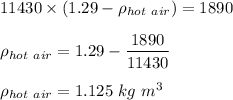
Therefore, the density of hot air in the balloon is 1.125 kg m³.