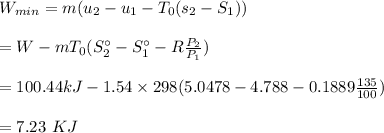Answer:
The answer is "
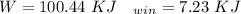 "
"
Step-by-step explanation:
Please find the complete question in the attached file.
From of the ideal gas relation that initial and the last temperatures were determined:
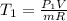
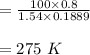
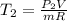
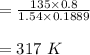
In the initial and final states, the internal energies for given temperatures are described from A-20 by means of intelmpolation and divided by the carlxon molar mass.
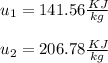
The real job is just the difference between internal energies:
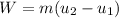
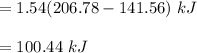
In the initial and final states, the zero entries are as determined as internal energies:
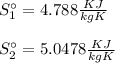
From its energy increase, the minimum work required is determined: