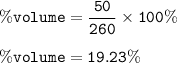The percent by volume of a solution : 19.23%
Further explanation
Given
50 ml of ethanol
210 ml of water
Required
The percent by volume of a solution
Solution
Percent Volume (% v/v) : volume (ml) of solute/100 ml of solution ⇒ ratio of the volume of the solute to total volume of the solution
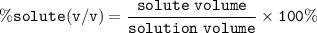
solute= Ethanol
solvent=water
Solution = solute+solvent
Total volume of the solution :

Percent by volume :