Given :
Mass of water, m = 2 grams.
The temperature of water drops from 31 °C to 29 °C .
The specific heat of water is 4.184 J/(g • °C).
To Find :
Amount of heat lost in this process.
Solution :
We know, heat lost is given by :
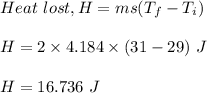
Therefore, amount of heat lost in this process is 16.736 J.