Answer:
8.51 x 10²¹ atoms
Step-by-step explanation:
Given parameters:
Mass of K = 0.551g
Unknown:
Number of atoms = ?
Solution:
To find the number of atoms in this given substance, we need to solve for the number of moles first.
Number of moles =
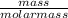
Now;
Molar mass of K = 39g/mol
Number of moles =
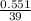 = 0.014mole
= 0.014mole
Therefore,
1 mole of a substance contains 6.02 x 10²³ atoms
0.014 mole of K will contain 0.014 x 6.02 x 10²³ = 8.51 x 10²¹ atoms