Answer:
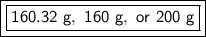
Step-by-step explanation:
To convert from grams to moles, we must use calcium's molar mass, which can be found on the Periodic Table of Elements.
- Calcium (Ca) Molar Mass: 40.08 g/mol
Now, we can use the molar mass as a ratio or fraction.
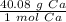
This fraction can be multiplied by the number of moles, which is 4.
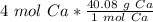
When we multiply, the moles of calcium will cancel each other out.
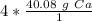
Since the fraction is over 1, we can just remove the denominator and write a simply multiplication equation.
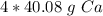
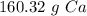
Depending on the teacher's instructions, the answer can be left as is or rounded.
If we round to the technically correct number of significant figures, it would be 1, because the original measurement of 4 moles has 1 sig fig. This would 200 grams, but that's a large amount to round.
We could also round to the nearest whole number. The 3 in the tenth place tells us to leave the whole number as it is. This would be 160 grams.
The answer could be 160.32 grams, 160 grams, or 200 grams, depending on rounding and significant figures.