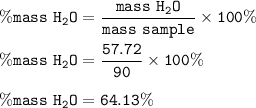The percent by mass of H₂O in the hydrated compound, Na₂CO₃.xH₂O = 64.13%
Further explanation
Given
90 g sample Na₂CO₃.xH₂O
32.28 g Na₂CO₃
Required
The percent by mass of H₂O
Solution
mass of H₂O = mass of sample - mass of the anhydrous salt, Na₂CO₃
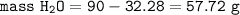
The percent by mass :