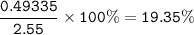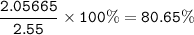The percentage composition of the alloy : 19.35% Zn, 80.65%Ag
Further explanation
Given
2.55 gram of an alloy
170 cc (170 ml=0.17 L) of Hydrogen gas(H₂)
Ar Zn = 65. H=1
Required
The percentage composition
Solution
Silver remained unsolved⇒no reaction
Hydrogen gas from reaction of Zn
Zn+2HCl⇒ZnCl₂ + H₂
mol H₂ at STP (STP⇒1 mol = 22.4 L) :
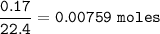
From equation, mol ratio Zn : H₂ = 1 : 1, so mol H₂ = 0.00759
Mass Zn :

Mass Silver :
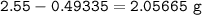
Percent composition :