The question is incomplete. The complete question is :
First Law of Thermodynamics - Sign Convention The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics is AU = Q-W. Here AU is the change in internal energy U of the system. Q is the net heat transferred into the system that is, Q is the sum of all heat transfer into (positive) and out of (negative) the system. W is the net work done by the system—that is, W is the sum of all work done by (positive) and on (negative) the system. We use the following sign conventions: if Q is positive, then there is a net heat transfer into the system; if W is positive, then there is net work done by the system. So positive Q adds energy to the system and positive W takes energy from the system. Thus AU = Q-W. Note also that if more heat transfer into the system occurs than work done, the difference is stored as internal energy. The first law of thermodynamics AU = 9 - W Ur-U, Heat Work System AU--W system w Qin: talu Qout:
The first law of thermodynamics AU = Q - W U-U Heat Work System AUQ-W Qin: ta Qout: - Wout: + WK w Win: - - volume expands t volume decreases o All answers can be positive or negative. (a) Suppose there is heat transfer of 42 ) into a system, while the system does 6 ) of work. Later, there is heat transfer of 22 J out of the system while 6 ) of work is done on the system. What is the net heat transfer? 20 Correct (100.0%) Submit What is the total work? Enter a number Submit (5 attempts remaining) What is the net change in internal energy of the system? Enter a number
What is the net change in internal energy of the system? Enter a number Submit (5 attempts remaining) (b) What is the change in internal energy of a system when a total of 140 J of heat transfer occurs out of (from) the system and 165 ) of work is done on the system? Enter a number Submit (5 attempts remaining) (c) An athlete doing push-ups performs 645 kJ of work and loses 440 kJ of heat. What is the change in the internal energy (in kJ) of the athlete? Enter a number Submit (5 attempts remaining) kJ (d) An athlete doing push-ups performs 690 kJ of work and loses 450 kJ of heat. Then he takes in 830 kJ of energy from eating food, What is the total change in the internal energy (in kJ) of the athlete? Enter a number kJ.
Solution :
a). Given :
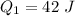 ,
,
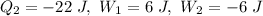
Net heat transfer
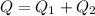
= 42 + (-22)
= 20 J
Total work
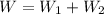
= 6 + (-6)
= 0 J
∵ ΔU = Q - W
= 20 - 0
= 20 J
This is the net change in the internal energy of the system.
b). ΔU = Q + W
= (-140) + (-165)
= -305 J
c). ΔU = Q + W
= (-440) + (645)
= 205 J
d). ΔU = Q + W
= (-450) + (690)
= 240 J