Answer:
0.2330 grams of solid KCl
Step-by-step explanation:
In order to find KCl in grams, we need to convert from moles to moles to grams..
However, we are given the volume and the molarity, so first we will use that to convert to moles of AgNO3 (silver nitrate) and then use that to convert to moles of KCl and finally to grams of KCl..
-----------------------------------------------------------------------------------------------------------------
Step 1 -> We need to convert milliliters to liters
(25.0 ml)(
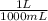 ) = 0.025 L
) = 0.025 L
Step 2 -> Convert from liters and molarity to just moles of AgNO3
(0.025 L)(0.125
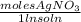 ) = 0.003125 moles
) = 0.003125 moles
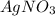
**Liters cancel out, so just multiply across...
Step 3 -> Now that we have moles of AgNO3, we will convert to moles of KCl
We will do this using the balanced equation
We see that in the balanced equation, for every mol of AgNO3 that reacts, one mol of KCl will react too. So we will set the dimensional analysis up like the following:
(0.003125 moles AgNO3) *
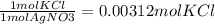
Step 4-> So now that we have moles of KCl, we will just convert to grams
We will do this using the molar mass (atomic mass) of KCl to convert to grams
0.00312 mol KCl *
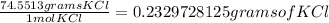
This can be rounded to 0.2330 grams of KCl