Answer:
The heat energy stored within them changes
Step-by-step explanation:
Heat energy is a function of the mass of a body, its specific heat capacity, and its temperature.
In this case, the bodies have the same specific heat capacity since they are made from the same material and the same temperature.
To get the quantity of heat stored in a body, we need to multiply all three parameters as shown below
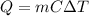
An increase in mass, through an increase in the amount of substance of a material present, will cause a corresponding increase in the heat energy stored within the material