Answer:
D. It is extremely reactive.
Step-by-step explanation:
Hello!
In this case, since potassium is an alkali metal, it is known those are extremely reactive because the energy required to ionize them is very low, it means they react so easily. For instance, even in the presence of water, potassium is able to react and form a purple flame as a product of the reaction:
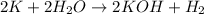
As well as potassium, the rest of the elements belonging to the alkali metals series are extremely reactive; therefore the answer is D. It is extremely reactive.
Best regards!