Answer:
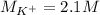
Step-by-step explanation:
Hello!
In this case, since we know the initial and final concentrations and the added volume of water, we can write:
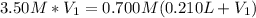
Which can be solved for the initial volume as follows:
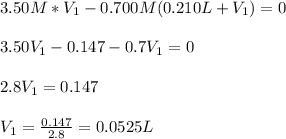
It means that the final volume is:
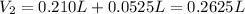
Next, we compute the moles of potassium phosphate in solution:
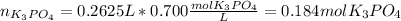
Then, since 1 mol of potassium phosphate has 3 moles of potassium (K's subscript), we compute the moles of potassium ions:
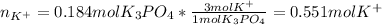
Finally, the concentration of potassium ions turns out:
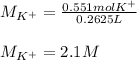
Regards!