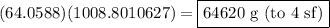Avogadro's law states that in a mole of any substance, there are
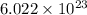 atoms. This means that in the given sample, there are
atoms. This means that in the given sample, there are
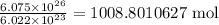
- The atomic mass of sulfur is 32.06 amu.
- The atomic mass of oxygen is 15.9994 amu.
So, the atomic mass of sulfur dioxide is
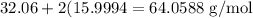
Therefore, the mass is: