Hey There!
_____________________________________
Answer:
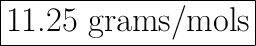
_____________________________________
Reaction:
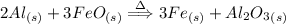
_____________________________________
DATA:
Mass of Aluminium: 27
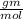
Mass of Ferous Oxide: 71.84
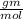
Aluminium: 7.5 Moles Given
How many moles of Iron (II) oxide(FeO)?
_____________________________________
SOLUTION:
As there are 2 moles of aluminium involved and 3 moles of ferrous oxide So,
2 moles Al and 3 moles FeO
7.5 moles Al and x moles FeO
For X moles:
X =
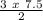
X=
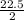
X= 11.25
_____________________________________
Best Regards,
'Borz'