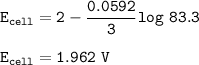E cell = 1.962 V
Further explanation
Given
E° cell = 2.00 V
Required
E cell at 298 K
Solution
The potential cell for nonstandard conditions we can use the Nernst equation
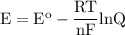
For temperature T = 298 K,
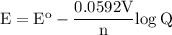
Reaction
3A(s) + B+3(aq) → 3A+(aq) + B(s)
Half Reaction
3A(s)⇒3A⁺(aq)+3e⁻
B⁺³(aq)+3e⁻⇒B(s)
n = 3 (3 electron transfer)
Q = the reaction quotient :
![\tt Q=([A^+]^3)/([B^(+3)])=(0.5^3)/(1.5.10^(-3))=83.3](https://img.qammunity.org/2021/formulas/chemistry/high-school/2h23t7n1x7w6iji1infdhvcvxjdlkv5fsm.png)
E cell :