Answer:
17.25g
Step-by-step explanation:
From our question, we have been asked to find the mass of water present.
The formula for finding specific heat is given by;
Q = mCp∆T
We have, Q = 0.507 J, ∆T = 0.007° C and we know that Cp of water is 4.2 J/g°C
Rearrange the formula making m the subject.
Which is given by;
m = (Q)/(Cp∆T)
Substituting for the known values
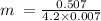
= 17.24489 g
= 17.25g