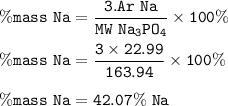The percent composition by mass for sodium (Na) in Sodium phosphate (Na₃PO₄) : 42.07%
Further explanation
Given
Compound of Sodium phosphate (Na₃PO₄)
Required
The percent composition by mass for sodium (Na)
Solution
The Comparative Law (Proust ) : compounds are formed from elements with the same Mass Comparison
MW Na₃PO₄ = 163,94 g/mol
Ar Na=22,989769g/mol