In container E, the pressure will be higher than container D
Further explanation
Given
Container with :
- the same particles
- the same temperature
- the different volume
Required
The higher pressure container
Solution
Boyle's Law stated that :
At a constant temperature, the gas volume is inversely proportional to the pressure applied
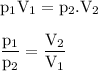
From the diagram (picture), it shows that the volume of container D is greater than container E, so the pressure applied to container D will be less than the pressure applied to container E