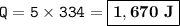Heat would be required : 1,670 J
Further explanation
Given
mass of H₂O=5 g
Required
Heat to melt
Solution
The heat to change the phase can be formulated :
Q = m.Lf (melting/freezing)
Lf=latent heat of fusion
The heat of fusion for water at 0 °C : 334 J/g
Input given values in formula :