Answer: The atomic mass of these two isotopes is 40.03 amu. The average mass is closer to actual amu of 58.69 for nickel as its percentage abundance is more.
Step-by-step explanation:
Mass of isotope 1 = 63.93
% abundance of isotope 1 = 0.93% =

Mass of isotope 2 = 57.93
% abundance of isotope 2 = (68.08)% =
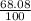
Formula used for average atomic mass of an element :
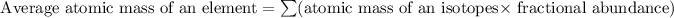
![A=\sum[(63.93)* (0.93)/(100))+(57.93)* (68.08)/(100)]]](https://img.qammunity.org/2021/formulas/chemistry/high-school/b1c6vhowmitlxnwm3apxpoiiwbi0dz6hjw.png)
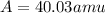
Therefore, the average atomic mass of nickel is, 40.03 amu. The average mass is closer to actual amu of 58.69 for nickel as its percentage abundance is more.