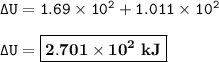The change in internal energy = 2.701 x 10² kJ
Further explanation
Given
Heat absorbed by system = 1.69 x 10² kJ
Work done on system = 1.011 x 10² kJ
Required
The change in Internal energy
Solution
We can use the first law of Thermodynamics :
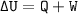
The sign rules for heat and work are set as follows:
• The system receives heat, Q +
• The system releases heat, Q -
• The system does work, W -
• the system accepts work, W +
Heat absorbed/receive heat from surrounding = Q+
Work done on the system = W+
and input the given values