Answer:
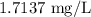
Step-by-step explanation:
Chlorine gas available = 27 lbs =
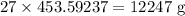
Amount of water used each day = 750000 gal =
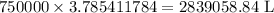
Rate at which chlorine gas used = 2.6 mg/L
Chlorine gas used
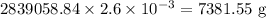
Residual content of chlorine =
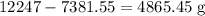
Concentration of the residual chlorine is given by
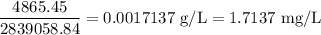
Concentration of the residual chlorine is
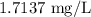 .
.