Answer: A change in the first second and third
Step-by-step explanation:
An electron in the lowest energy state is called as ground state and is also known as stable energy level. An electron in the upper energy state is called as excited state and is also known as unstable energy level.
Electronic configuration represents the total number of electrons that a neutral element contains. The electrons are filled according to Afbau's rule in order of increasing energies. As the atomic number of sodium is 11. The electronic configuration of sodium will be represented in ground state as:
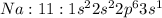
Thus any electron if occupies a higher shell, the atom is said to be in excited state.