Answer:
The half-life is
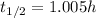
Step-by-step explanation:
Using the decay equation we have:
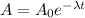
Where:
- λ is the decay constant
- A(0) the initial activity
- A is the activity at time t
We know the activity decrease by a factor of two in a one hour period (t = 1 h), it means that
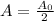
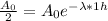
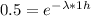
Taking the natural logarithm on each side we have:
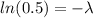
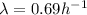
Now, the relationship between the decay constant λ and the half-life t(1/2) is:
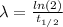
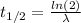
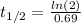
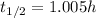
I hope it helps you!