Answer:
0.16mole
Step-by-step explanation:
To solve this problem, we are going to assume that the number of moles of carbon to be determined is that at STP, standard temperature and pressure.
The number of moles of a substance at STP is given as;
Number of moles =
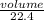
Given volume = 3.5L
Now, insert the parameters;
Number of moles =
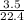 = 0.16mole
= 0.16mole