Answer:
3.69 g
Step-by-step explanation:
Given that:
The mass m = 325 g
The change in temperature ΔT = ( 1540 - 165)° C
= 1375 ° C
Heat capacity
 = 0.490 J/g°C
= 0.490 J/g°C
The amount of heat required:
q = mcΔT
q = 325 × 0.490 × 1375
q = 218968.75 J
q = 218.97 kJ
The equation for the reaction is expressed as:
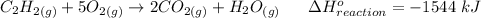
Then,
1 mole of the ethyne is equal to 26 g of ethyne required for 1544 kJ heat.
Thus, for 218.97 kJ, the amount of ethyne gas required will be:
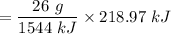
= 3.69 g