Answer:
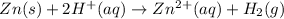
Step-by-step explanation:
Hello!
In this case, since writing the net ionic equation implies the complete molecular equation, we should start by:
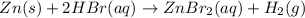
Next, we represent the ionization of the aqueous species:
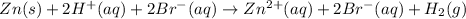
Whereas the spectator ions, those at both reactants and products are cancelled out so the net ionic equation is obtained:
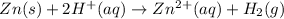
Best regards!